Abstract
Introduction: Multiple structural elements are known that recruit kinases to sites of active signal transduction (e.g. lipid rafts) within the plasma membrane. For instance, pleckstrin homology (PH) domains and the conserved intracellular loop (CIL) motifs recently discovered by us (Lee et al., Nature 2021) direct kinase molecules (Src, BTK, AKT and PI3K) to large signaling clusters in lipid rafts to amplify normal or oncogenic B-cell signaling. While concepts of site-specific recruitment of kinases to initiate signaling are well established, little is known about spatial control of inhibitory phosphatases and how they are directed to sites of maximal signaling strength.
Results: Here we discovered the cytoplasmic tail of CD25 as an essential structural element for site-specific recruitment of inhibitory phosphatases. CD25-mediated shuttling of inhibitory phosphatases balances oncogenic signaling strength and is essential for cell-survival in B-cell malignancies, including B-ALL, CLL and mantle cell lymphoma. This was unexpected because CD25 (IL2RA) is known as one of three chains of the Interleukin-2 (IL2) receptor on T- and NK-cells. Studying clinical outcome and gene expression in multiple clinical cohorts, we found CD25 as a top-ranking predictor of poor clinical outcome in patients with B-ALL, mantle cell lymphoma and CLL.
Our mechanistic experiments revealed that CD25 expressed on B-cells is monomeric. Unlike T- and NK-cells, CD25 expressed on activated or transformed B-cells does not form a heterotrimeric IL2 receptor with the IL2Rb and common g-chain. Instead of contributing to IL2-signaling, CD25 showed dynamic recruitment either to the B-cell receptor (BCR) or transforming oncogenes within lipid rafts. Conditional deletion of CD25 at earliest stages of B-cell development in mice resulted in profound B-cell defects. However, these defects were not replicated by IL2-deficiency and B-cell development in Il2-/- mice was normal.
Inducible deletion of CD25 in genetic models for B-ALL and mantle cell lymphoma revealed an essential function of CD25 in maintaining homeostasis of signaling strength. In the absence of CD25, B-ALL and mantle cell lymphoma cells showed autonomous Ca 2+ oscillations, constitutive activation of Src-family kinases, BTK and ERK as well as the AMPK energy-stress sensor. CD25-deficient B-ALL and mantle cell lymphoma cells initially showed increased proliferation but rapidly died from exhaustion. Consistent with massive accumulation of p53, CD25-deficient B-ALL cells failed to form colonies and to initiate leukemia in transplant-recipient mice.
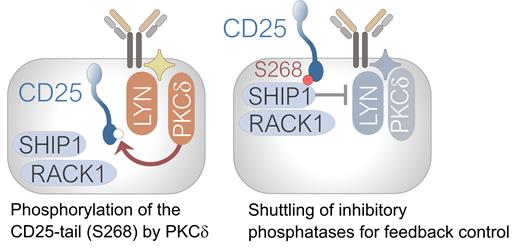
Our mechanistic studies focused on the short (13 residues) cytoplasmic tale of CD25, which features a prominent serine/threonine kinase substrate motif. In vitro kinase assays for 62 candidate serine/threonine kinases revealed PKCd as top-ranking kinase and the serine residue 268 in the tail of CD25 as its principal substrate. The proximity-based labeling assays (BioID) revealed that the CD25 tail recruits PKCd and its adapter RACK1, which scaffold the inhibitory phosphatases SHIP1 and SHP1 for site-specific activation in lipid rafts (see Schematic). Consistent with these results, phospho-proteomic studies revealed that inducible deletion of CD25 resulted in acute inactivation of SHIP1 and SHP1 as well as the inhibitory RACK1 scaffold. Multiscale molecular dynamics (MD) simulations revealed that PKCd-mediated phosphorylation of the CD25-tail (S268) tightened interactions with RACK1 and SHIP1 and SHP1 phosphatases, whereas these interactions were destabilized when the CD25-tail was mutated (S268A).
Conclusions: Mechanisms of site-specific recruitment of kinases for signal amplification have been extensively studied. However, structural elements that direct inhibitory phosphatases to sites of maximal signaling activity for feedback control remained largely unknown. Here we discovered a PKCd substrate motif in the cytoplasmic tail of CD25 (S268) as a central element to recruit inhibitory phosphatases, scaffolded by RACK1, to curb excessive signaling activity and energy expenditure. High expression levels of CD25 enable higher baseline activity of oncogenic signaling and are associated with poor patient-outcome. Our preclinical studies based on CD25-antibody-drug-conjugates (ADC) revealed that CD25 as a novel target in refractory B-ALL and mantle cell lymphoma.
Weinstock: SecuraBio: Consultancy; ASELL: Consultancy; Bantam: Consultancy; Abcuro: Research Funding; Verastem: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; AstraZeneca: Consultancy; Travera: Other: Founder/Equity; Ajax: Other: Founder/Equity.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal